The drug development process is a complex journey that takes a new drug from the laboratory to the patient. It involves a series of rigorous stages to ensure that the drug is safe, effective, and beneficial to the patient.
The Importance of Drug Development
Drug development is crucial in the pharmaceutical industry. It’s the process that brings new treatments to patients who need them, improving health outcomes and often saving lives. This is particularly important when considering pharmaceutical trials for Alzheimer’s, where new treatments can significantly improve patients’ quality of life.

Stages of Drug Development
The drug development process typically involves the following stages:
1. Discovery and Development
Researchers discover new drugs through various methods such as genetic manipulation, drug repurposing, and computer modeling. Once a promising compound is identified, it undergoes laboratory testing for several years to study its safety and effectiveness. This stage is crucial in drug formulation.
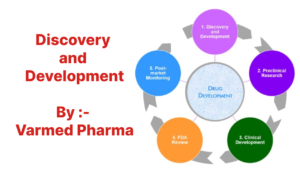
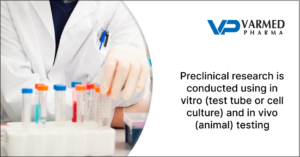
2. Preclinical Research
Before testing a drug on humans, preclinical research is conducted using in vitro (test tube or cell culture) and in vivo (animal) testing. This stage assesses the safety of the drug and how it works biologically. It’s also where drug delivery methods for insulin and other medications are often developed.
3. Clinical Trials
If the results of preclinical research are promising, the drug moves to clinical trials, which are conducted in three phases. Each phase involves testing the drug on a larger group of people.
4. FDA Review
If the clinical trials are successful, a New Drug Application (NDA) is submitted to the Food and Drug Administration (FDA) for review. The FDA evaluates the drug’s safety, efficacy, and manufacturing process1. This is where pharmaceutical quality control comes into play, ensuring that all drugs meet the highest standards.
5. FDA Post-Market Safety Monitoring
Once a drug is approved and made available to patients, the FDA continues to monitor its safety to identify any adverse events1. This includes monitoring the use of prescription drugs.
The Future of Drug Development

With advancements in technology and a better understanding of diseases at a molecular level, the dug development process is continually evolving. Personalized medicine, targeted therapies, and the use of artificial intelligence are some of the trends shaping the future of drug development.
Conclusion
Understanding the drug development process is crucial for anyone involved in the pharmaceutical industry. It not only helps in the creation of new drugs but also ensures that the drugs are safe and effective for patients. This is particularly important for those looking into pharma franchise opportunities or seeking to establish pharmaceutical franchise companies in India.

Стильные заметки по подбору крутых видов на любой день.
Статьи стилистов, новости, все новинки и мероприятия.
https://mvmedia.ru/novosti/122-gde-luchshe-pokupat-originalnye-brendovye-veshchi-kak-vybrat-nadezhnye-magaziny-i-platformy/